DRUG DISCOVERY
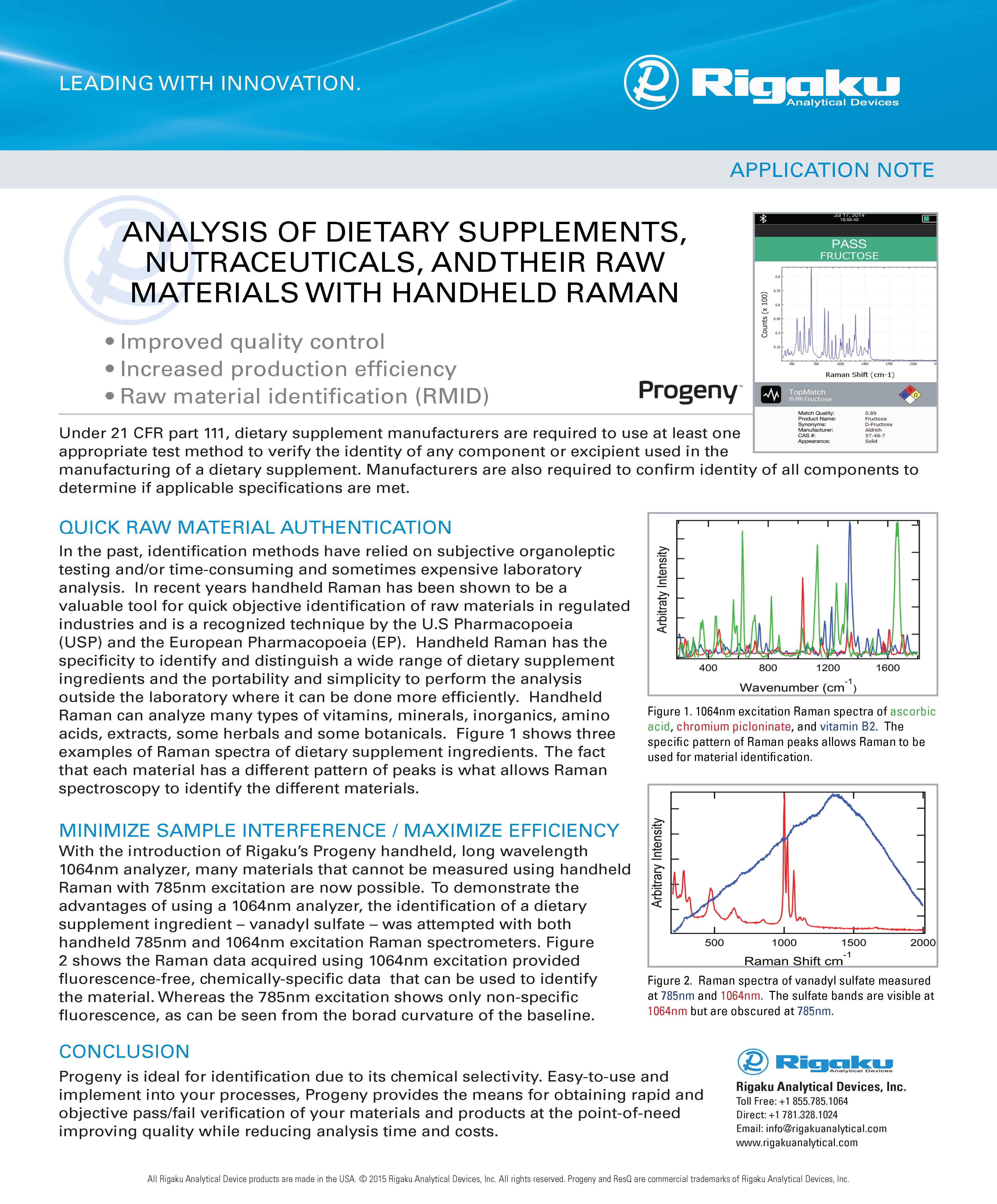
DRUG DISCOVERY
DATA INTEGRITY
GLOBAL COMPLIANCE
QUALITY ASSURANCE
DRUG DISCOVERY AND DEVELOPMENT PROCESS
To find and develop active pharmaceutical compounds that are safe, effective, and suitable for manufacture, requires a surprising breadth of research and investigation. According to the current state of chemical and biological sciences utilized for pharmaceutical development, some 5,000–10,000 chemical compounds have passed through laboratory screening for each new medicine licensed for human use.
Around 250 of the 5,000–10,000 compounds examined will go into preclinical research, and 5 will go into clinical testing. The entire process of developing a medicine, from discovery to marketing, can take 10 to 15 years. This section introduces the typical flow of the discovery process for New Molecular Entities (NMEs) within the pharmaceutical industry.
The Discovery of NMEs results from the efforts of a diverse group of public and commercial entities involved in the development and manufacturing of active pharmaceutical ingredients (APIs) and drug products. Many publicly financed institutions do basic research in fields including chemistry, biochemistry, physiology, microbiology, and pharmacology as part of this process.
Rather than focusing on the production of a product or technology, basic research typically aims to gain a better knowledge of natural chemicals or physiological processes. This allows researchers at both public and private institutions to apply this fundamental knowledge to the discovery of new NMEs matched to specific metabolic pathways or protein targets.
Scientists and medics working in a range of research institutions and universities perform the first steps in this procedure. Their findings are published in peer-reviewed scientific and medical journals. These findings aid in the identification of potential new targets for NME development related to specific disease states. A drug receptor, an enzyme, a biological transport route, or any other mechanism engaged in body metabolism could be suitable targets.
Almost all of the complex biochemical operations in the human body and within all of our cells are controlled by enzymes. Characterization of the three-dimensional enzyme structure and key active sites on the enzyme are major NME discovery and structure-based drug design components. High-throughput protein structure determination enables the screening of large numbers of candidate NMEs and significantly reduces discover timelines.
Once suitable targets and NMEs have been identified, Pharmaceutical corporations carry out or direct the majority of the remaining work involved in drug research and development.
Rigaku offers a range of screens, instrumentation, and software to enable and facilitate the drug discovery process. From protein crystallization kits and screens to high-throughput protein and small-molecule crystal structure elucidation, Rigaku leads the way. With innovative small-angle X-ray scattering (SAXS) systems specifically designed for automated protein analysis and unique pair distribution function (PDF) methods for small molecules, Rigaku supports the future of drug discovery.
A DAY OF EDUCATION AND INSIGHTS FOR PHARMACEUTICAL PROFESSIONALS THROUGHOUT THE PRODUCT DEVELOPMENT LIFECYCLE
Pharmalytical Summit 2021 is a one-day virtual event exploring the technologies, analysis, and innovation that goes into bringing the pharmaceuticals allowing humanity to live longer, fuller, healthier lives.
The day features presentations and discussions with industry leaders and experts who share insights and education to help bring these life-changing products into the world.
Join us on May 26th, 2021 from 8 AM to 8 PM CDT.
RELATED APPLICATIONS
The Use of Atomic Pair Distribution Function Analysis to Verify Molecular Structure for Amorphous Materials
Small Angle X-ray Scattering Approaches for Pharmaceutical Studies
Thermal Analysis for Pharmaceutical Applications
A Non-Destructive XRF Technique for Rapid and Easy Screening of Residual Catalysts in API's & Intermediates
INDUSTRY-LEADING SUPPORT

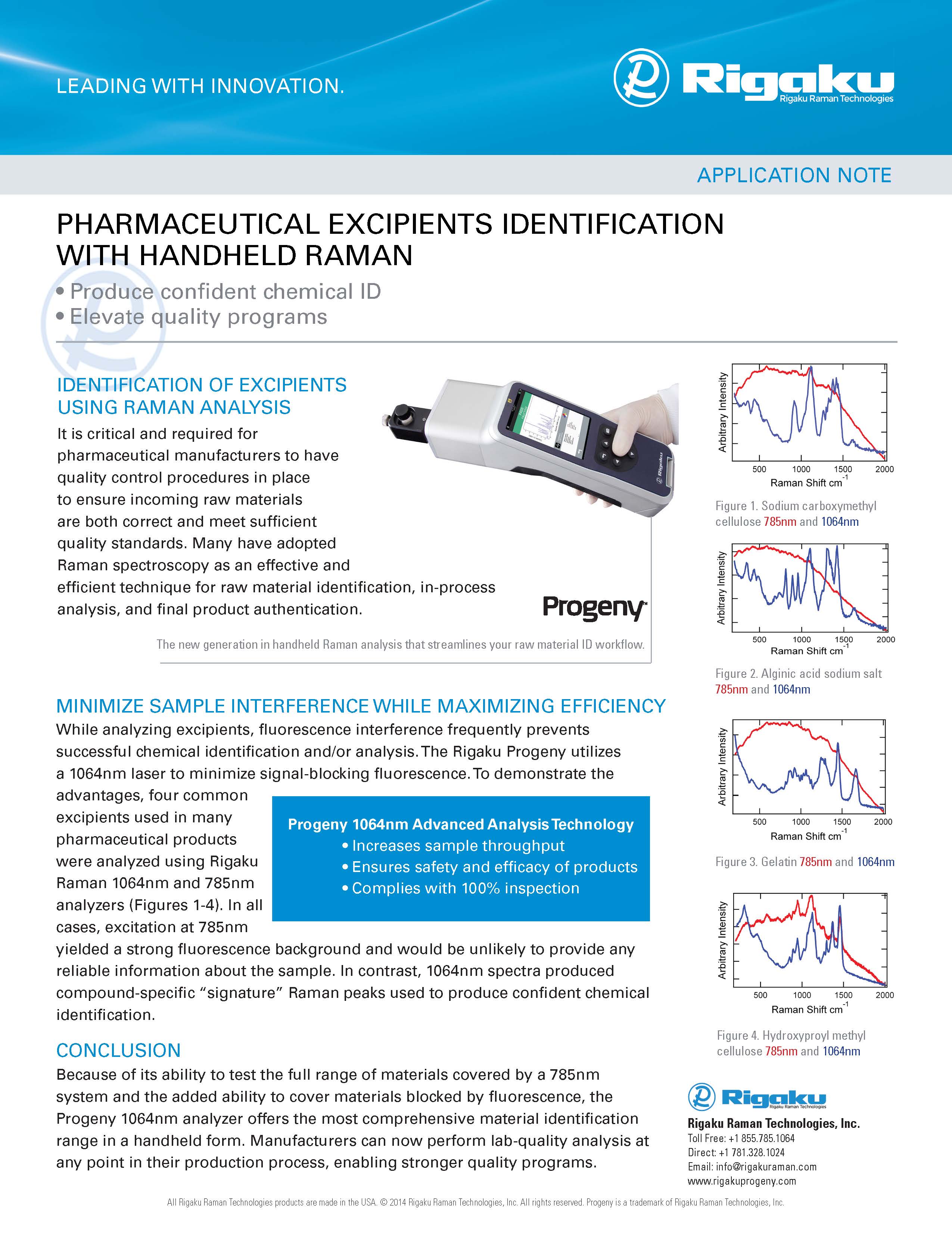
"We use Progeny at every point in our manufacturing."

"Another thing I like about Progeny is the software as it relates to data integrity, ALCOA and those type of principals that you’re operating under a GMP environment.”

“I came at this with very little analytical background…and I found the instrument really easy to use…”

"The fact that we can continue to add new materials to the library is a huge benefit and the device is now also being used to establish ID methods in new product ingredients."
MADE IN THE USA
FOCUSED CUSTOMER SUPPORT
RECOMMENDED PRODUCT
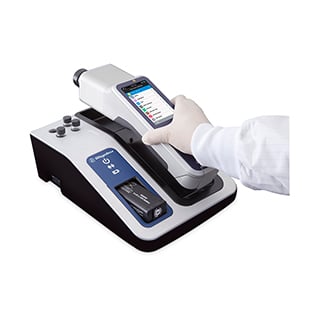
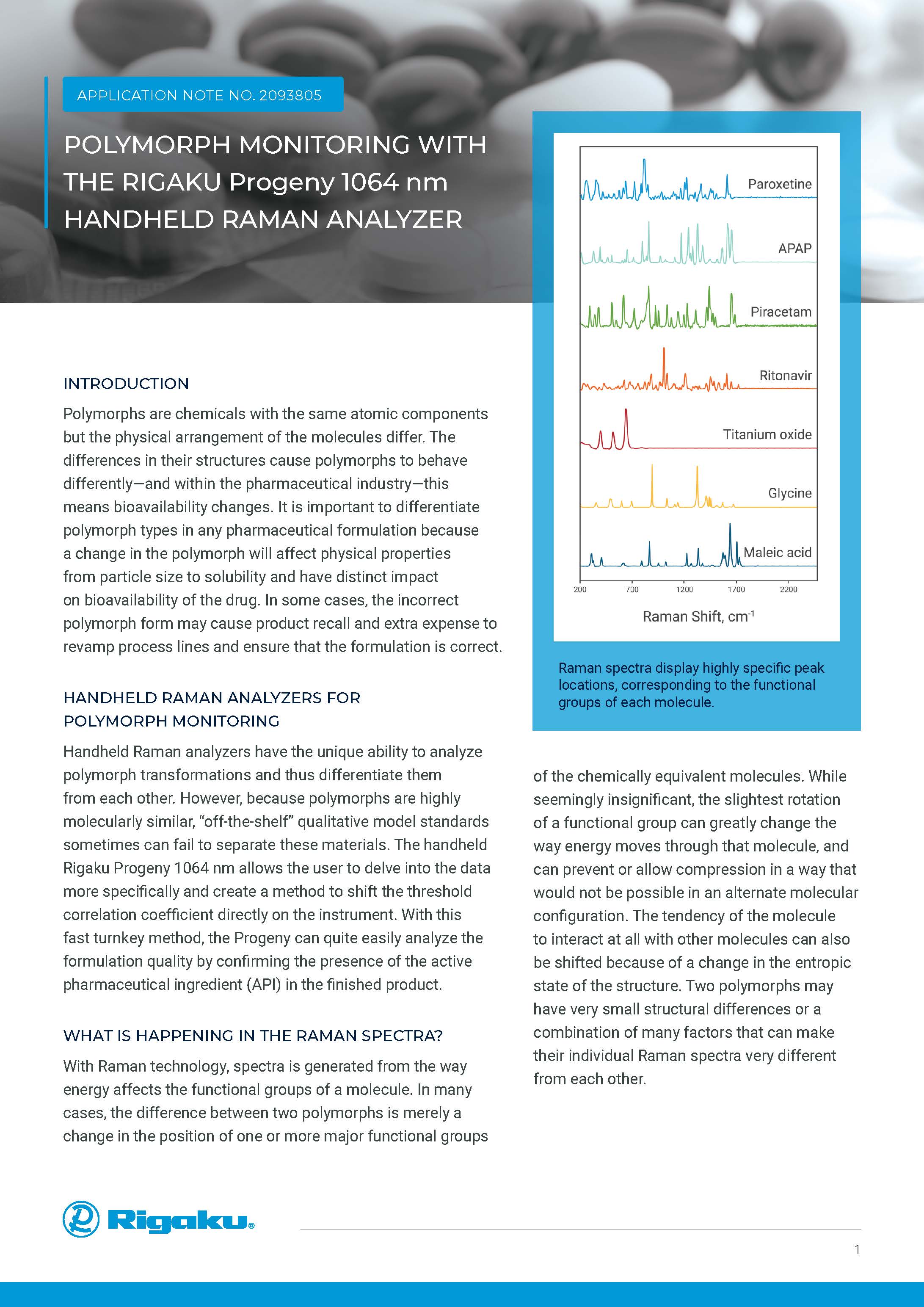
PROGENY HANDHELD 1064 nm Raman

Versatile Spectrometer for Raw Material Identification and Verification
LEARN MORECUTTING-EDGE TECHNOLOGY
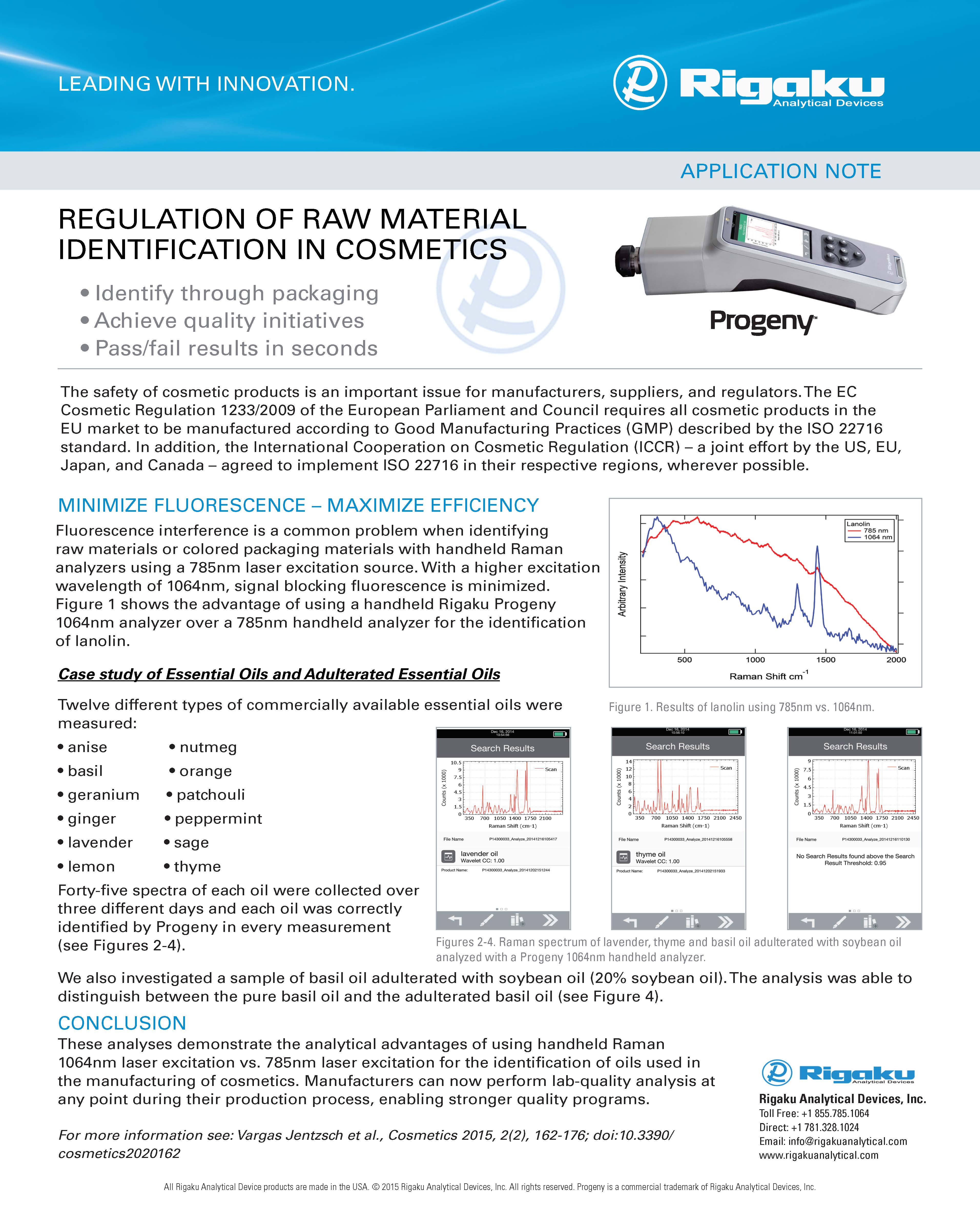
HANDHELD 1064 nm RAMAN SPECTROSCOPY
FEATURES & BENEFITS
- NONDESTRUCTIVE
- ANALYZE SOLIDS, LIQUIDS, POWDERS, GELS, PASTES AND MORE
- SCAN THROUGH COLORED PACKAGING
- ANALYZE COLORED SUBSTANCES
- RESULTS < 1 MINUTE
- MINIMIZED FLUORESCENCE
HOW WE WORK WITH YOU
TRUSTED PARTNER
Based in Wilmington, Massachusetts, USA since 2011, Rigaku is a pioneer in handheld 1064 nm Raman and LIBS-based technology for materials analysis. With thousands of units in use globally, our portfolio offers the most versatile solution for use in safety and security against chemical threats, pharmaceutical manufacturing, and in the recycling and quality control of metal alloys.
We strive to deliver quality, reliability and engaged expertise to our customers. We are dedicated to continuous product development efforts to deliver mission-critical enhancements to performance and functionality, while delivering reliable and cost-effective solutions for results, anywhere.
Rigaku Analytical Devices is an ISO 9001:2015 certified facility.
RESOURCES
- BROCHURE
- SPECIFICATION SHEET
- APPLICATION NOTES
- FAST FACTS
- WHITE PAPERS
- CASE STUDIES
- ACCESSORIES







_Page_1.jpg)