POLYMORPHISM IN PHARMACEUTICAL DRUG DEVELOPMENT
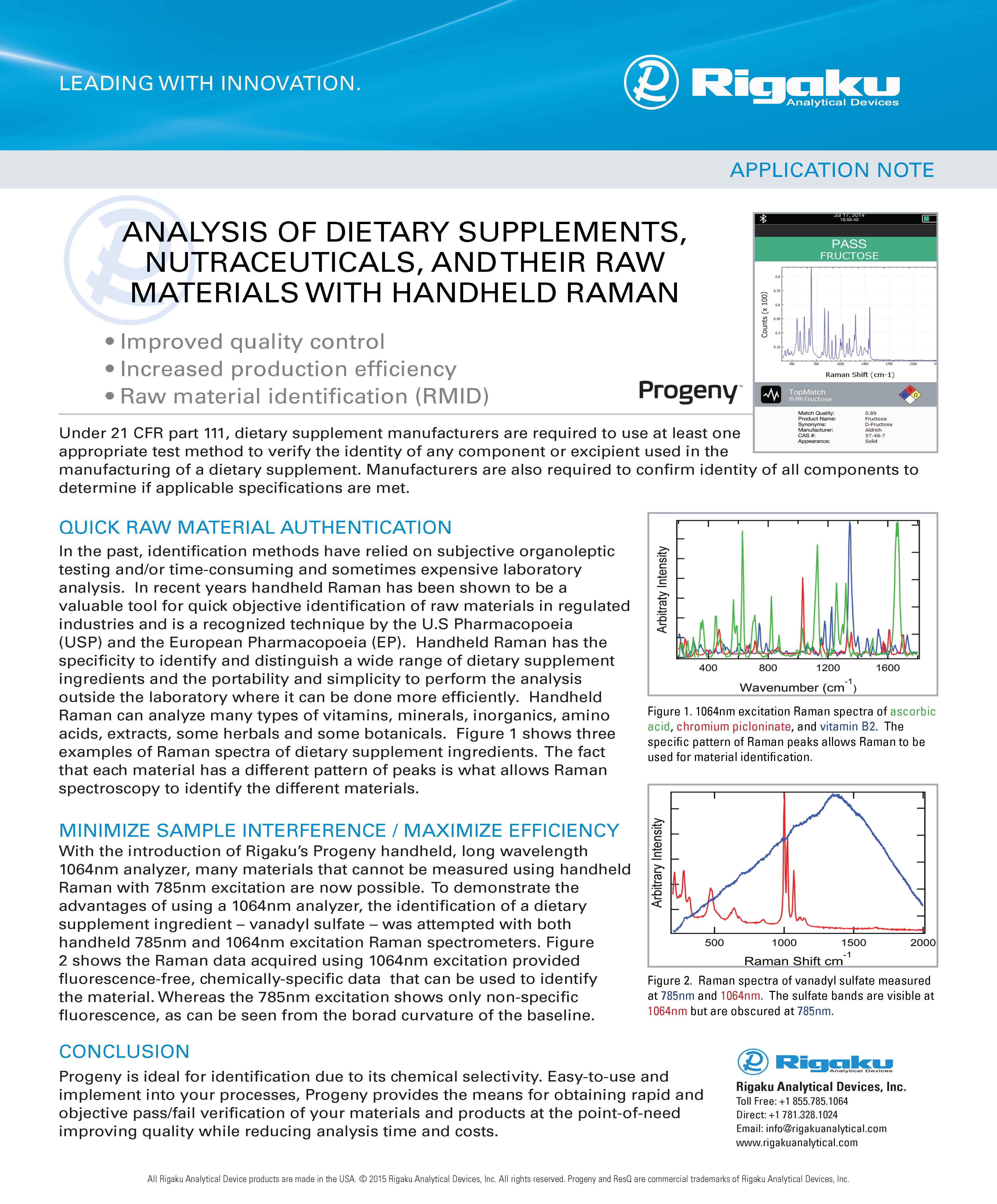
DRUG PREFORMULATION
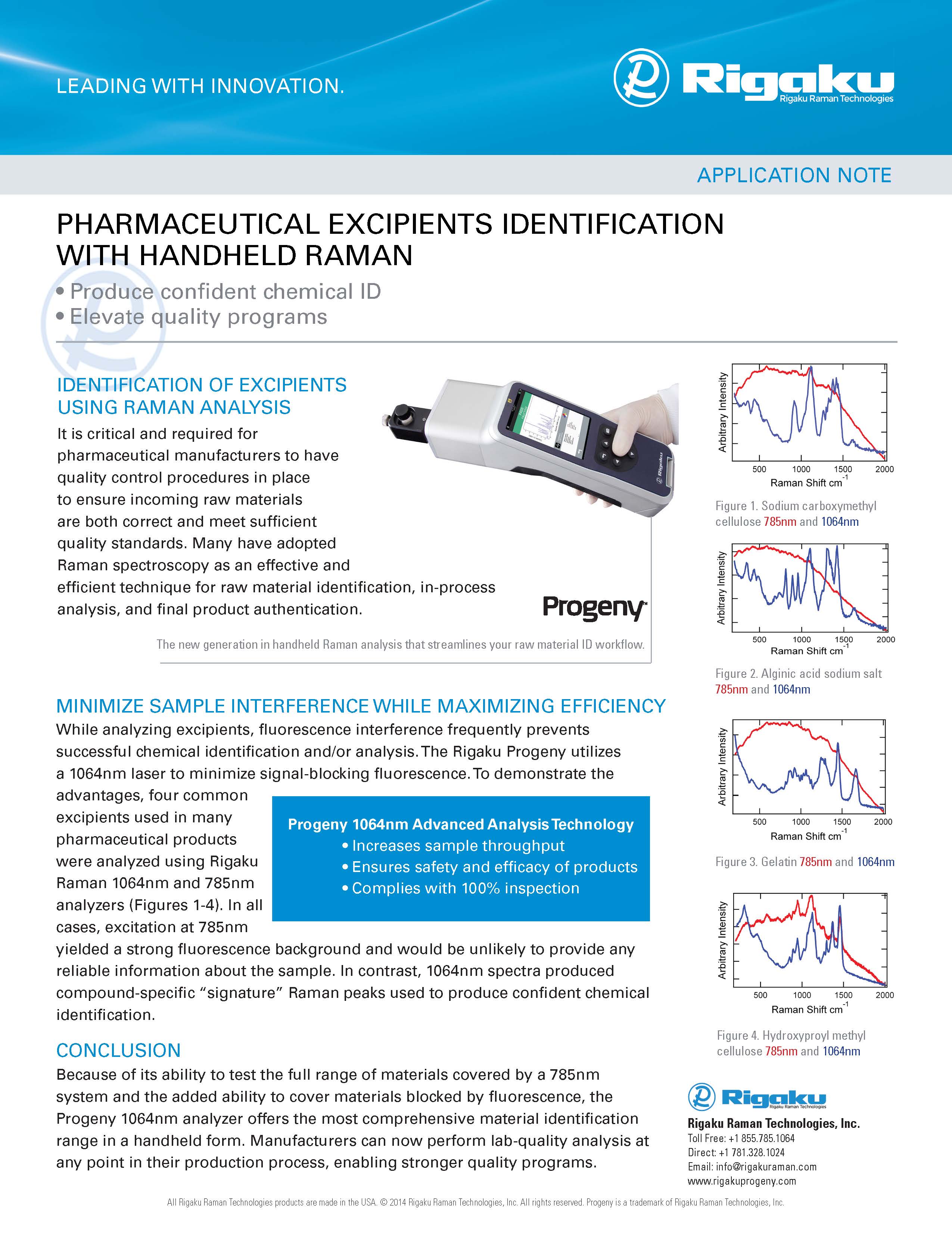
DATA INTEGRITY
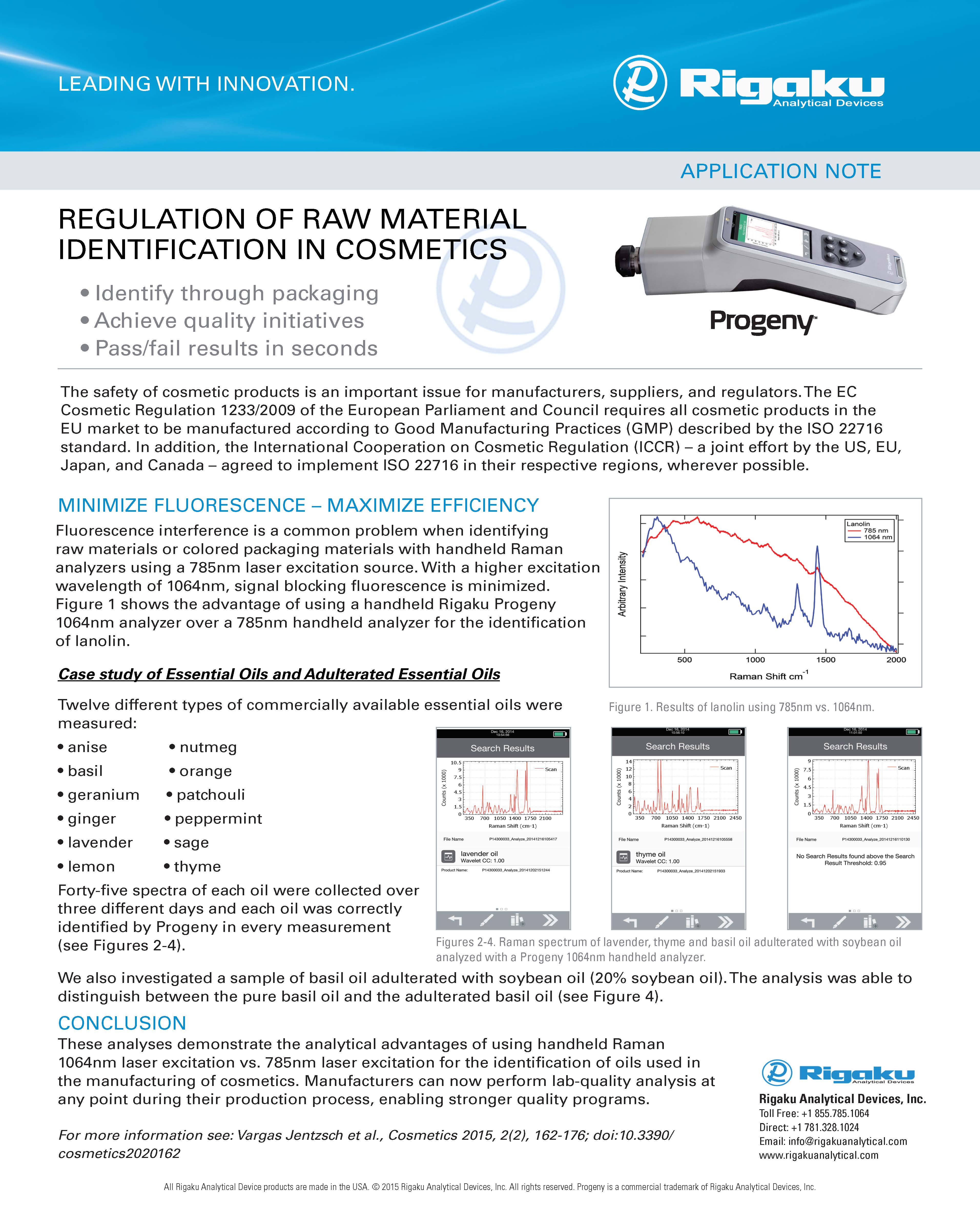
GLOBAL COMPLIANCE
QUALITY ASSURANCE
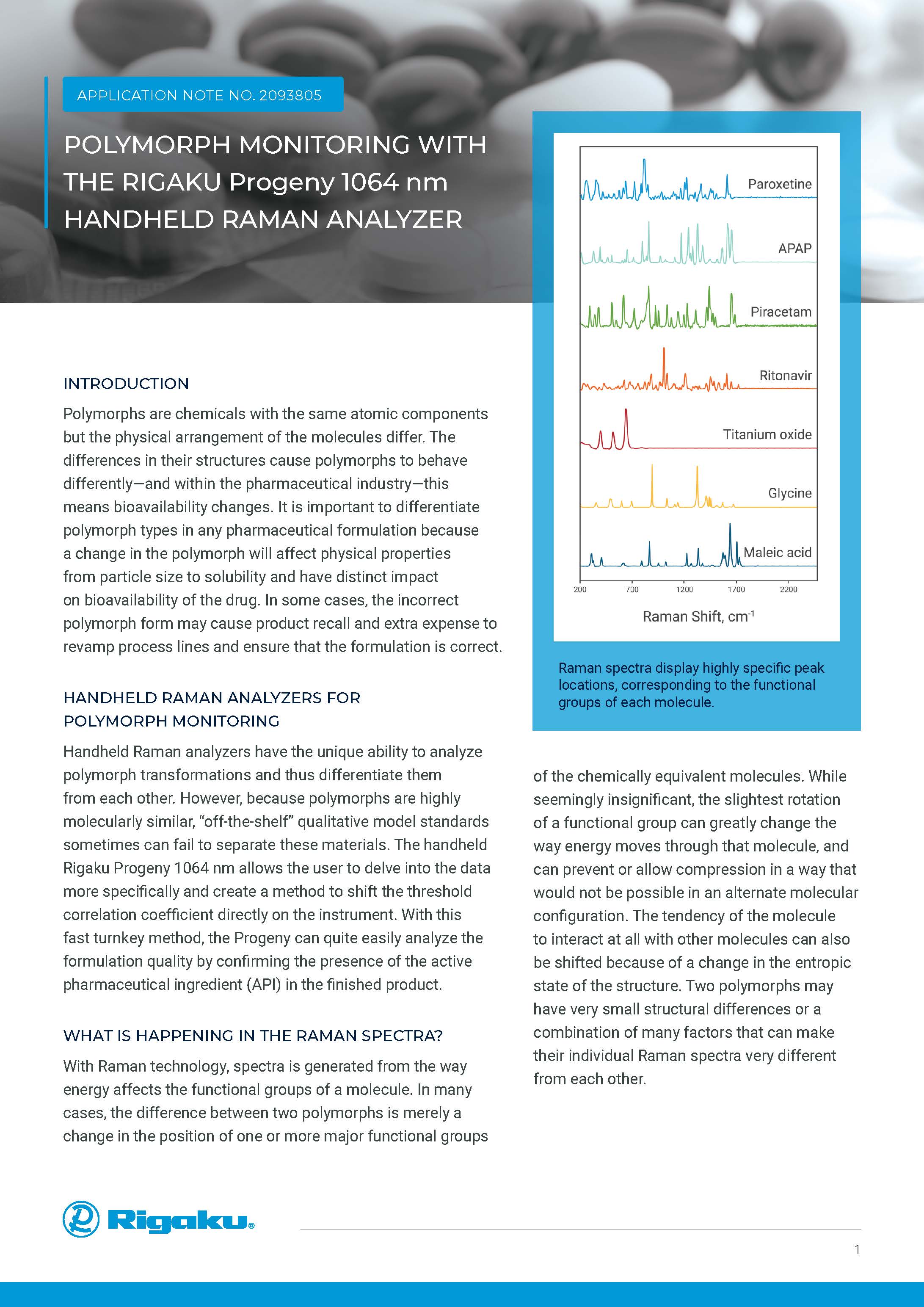
Polymorphs
From a material science perspective, polymorphs are crystalline forms of the same molecular entity in which the molecules in the crystal lattice have diverse configurations and/or conformations. As a result, their physico-chemical properties, such as solubility, stability, bulk density, crystalline habit and flow characteristics, change.
Under the regulatory definition, a polymorph is essentially a solid form delivery mechanism for the active molecule and can include amorphous forms and hydrates/solvates. Hydrates and solvates are multicomponent single phase solid forms (usually crystalline) containing the active molecule. In a similar fashion, cocrystals and amorphous solid dispersions (ASDs) can also be considered to be multicomponent single phase solid forms containing the active molecule. While cocrystals have recently been included into the regulatory polymorph landscape, ASDs are more usually considered to be an intermediate. Under the regulatory definition, most active molecules will exhibit polymorphism of some degree.
Salt forms incorporating an ionized form or the parent molecule are considered to be a different molecular entity as the parent molecule has been modified (ionized) during salt formation. Salt forms may also be polymorphic.The drive to keep costs low in the early development stages, combined with the need to move swiftly through development and the high attrition rate of candidates, has sparked interest in a phase-appropriate polymorph screening technique for small molecule candidates. Screening operations become more comprehensive as resources become available and technical needs alter in a phase-appropriate methodology, which is an iterative process. Limited screens are employed during early development to establish an appropriate solid form that would allow a speedy advance to the next milestone.
One of the first steps within pre-formulation is an abbreviated polymorph screen to identify a stable crystalline solid form that can be used for purification purposes and initial performance testing. A more extended screen is often required during early-stage formulation as the formulation tactics will depended on the polymorphic landscape. As a result, even in this early stage, it's critical to comprehend the various polymorphic forms, their stability and qualities, as well as their ability to convert from one form to another. The formulation approach would then be centered on preventing the selected polymorph from converting and ensuring its stability during the product's clinical stability or shelf life. As more purified material and resources become available a later stages in development, after clinical proof-of-concept, a complete screening can be undertaken to locate all solid forms for intellectual property protection and determination of the best solid form for commercialization.
A thorough polymorph screening approach should evaluate a range of variables that could impact the nucleation and growth kinetics of various crystalline forms. These include a wide range of solvents systems including mixed organic/aqueous solvents with various water activity levels, and various crystallization mechanisms such as: slurry ripening, rapid and slow cooling, and evaporative crystallization. Additional studies would include solvent and anti-solvent additives along with temperature variation. Experiments to examine process-induced polymorph change, such as API micronization, wet granulation, tableting, and generation of novel solvate with excipients, should also be included.
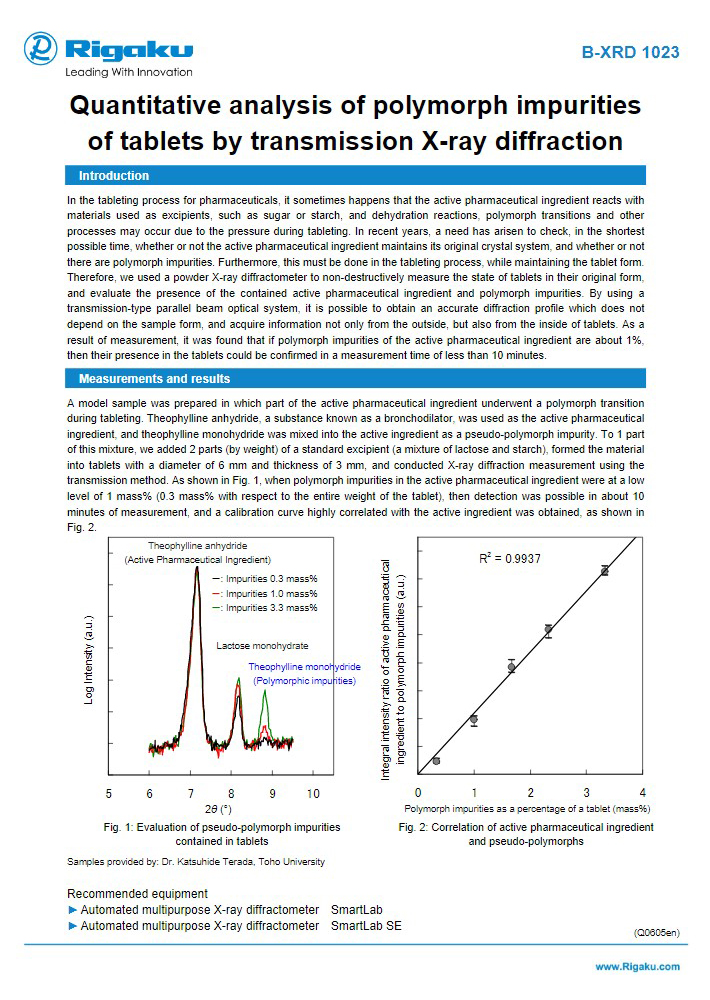
During the investigation of different solid forms, analytical chemistry plays a vital role. Utilizing techniques such as X-ray Diffraction (XRD), Raman/IR spectroscopy and Thermal analysis (such as DSC) for structural characterization of the different solid forms and an understanding of the relationships between the different polymorphs.
Polymorph screening is required for an active molecule in order to identify the distinct crystalline forms, hydrates/solvates, and cocrystals that can exist, as well as to determine their thermodynamic inter-relationships. Rigaku provides a range of customizable diffraction, spectroscopic and thermal analysis systems with performance to match abbreviated and comprehensive screens.

A DAY OF EDUCATION AND INSIGHTS FOR PHARMACEUTICAL PROFESSIONALS THROUGHOUT THE PRODUCT DEVELOPMENT LIFECYCLE
Pharmalytical Summit 2021 is a one-day virtual event exploring the technologies, analysis, and innovation that goes into bringing the pharmaceuticals allowing humanity to live longer, fuller, healthier lives.
The day features presentations and discussions with industry leaders and experts who share insights and education to help bring these life-changing products into the world.
Join us on May 26th, 2021 from 8 AM to 8 PM CDT.
RELATED APPLICATIONS
RELATED PRESENTATION - POLYMORPH ROUND TABLE FROM PHARMALYTICAL SUMMIT 2021
INDUSTRY-LEADING SUPPORT

"We use Progeny at every point in our manufacturing."

"Another thing I like about Progeny is the software as it relates to data integrity, ALCOA and those type of principals that you’re operating under a GMP environment.”

“I came at this with very little analytical background…and I found the instrument really easy to use…”

"The fact that we can continue to add new materials to the library is a huge benefit and the device is now also being used to establish ID methods in new product ingredients."
MADE IN THE USA
FOCUSED CUSTOMER SUPPORT
RECOMMENDED PRODUCT
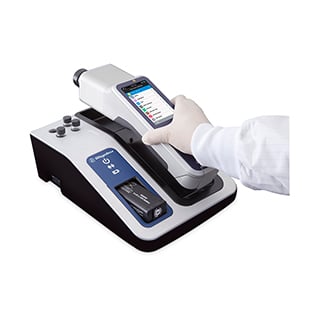
PROGENY HANDHELD 1064 nm Raman

Versatile Spectrometer for Raw Material Identification and Verification
LEARN MORECUTTING-EDGE TECHNOLOGY
HANDHELD 1064 nm RAMAN SPECTROSCOPY
FEATURES & BENEFITS
- NONDESTRUCTIVE
- ANALYZE SOLIDS, LIQUIDS, POWDERS, GELS, PASTES AND MORE
- SCAN THROUGH COLORED PACKAGING
- ANALYZE COLORED SUBSTANCES
- RESULTS < 1 MINUTE
- MINIMIZED FLUORESCENCE
HOW WE WORK WITH YOU
TRUSTED PARTNER
Based in Wilmington, Massachusetts, USA since 2011, Rigaku is a pioneer in handheld 1064 nm Raman and LIBS-based technology for materials analysis. With thousands of units in use globally, our portfolio offers the most versatile solution for use in safety and security against chemical threats, pharmaceutical manufacturing, and in the recycling and quality control of metal alloys.
We strive to deliver quality, reliability and engaged expertise to our customers. We are dedicated to continuous product development efforts to deliver mission-critical enhancements to performance and functionality, while delivering reliable and cost-effective solutions for results, anywhere.
Rigaku Analytical Devices is an ISO 9001:2015 certified facility.
RESOURCES
- BROCHURE
- SPECIFICATION SHEET
- APPLICATION NOTES
- FAST FACTS
- WHITE PAPERS
- CASE STUDIES
- ACCESSORIES











_Page_1.jpg)