PHARMACEUTICAL EXCIPIENTS IDENTIFICATION
It is critical and required for pharmaceutical manufacturers to have quality control procedures in place to ensure incoming raw materials are both correct and meet sufficient quality standards. Many have adopted Raman spectroscopy as an effective and efficient technique for raw material identification, in-process analysis, and final product authentication.
MINIMIZE SAMPLE INTERFERENCE WHILE MAXIMIZING EFFICIENCY
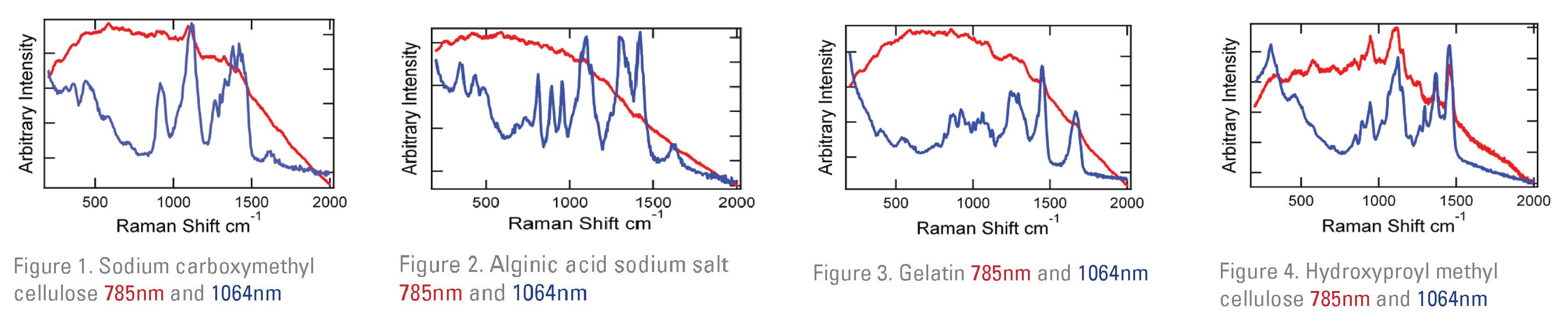
While analyzing excipients, fluorescence interference frequently prevents successful chemical identification and/or analysis. The Rigaku Progeny utilizes a 1064 nm laser to minimize signal-blocking fluorescence. To demonstrate the advantages, four common excipients used in many pharmaceutical products were analyzed using 1064 nm and 785 nm analyzers (Figures 1-4). In all cases, excitation at 785 nm yielded a strong fluorescence background and would be unlikely to provide any reliable information about the sample. In contrast, 1064 nm spectra produced compound-specific “signature” Raman peaks used to produce confident chemical identification.

CONCLUSION
Because of its ability to test the full range of materials covered by a 785 nm system and the added ability to cover materials blocked by fluorescence, the Progeny 1064 nm analyzer offers the most comprehensive material identification range in a handheld form. Manufacturers can now perform lab-quality analysis at any point in their production process.
RECOMMENDED PRODUCT
Progeny Handheld 1064nm Raman

