MECHANOCHEMICAL EFFECT OF CAFFEINE BY DSC
Introduction
Mechanical pulverizing methods for sample preparation such as ball milling or hand grinding have been used in different materials including pharmaceuticals. However, the use of mechanical energy during sample preparation can significantly affect the thermal behavior of a pharmaceutical material. Caffeine is often used in combination with other drugs such as stimulants, pain relievers, diuretics, cold remedies, etc. This drug is known to exist in two polymorphs, a Form II or β-form which is stable at ambient temperature and pressure; and a Form I or α-form that can be observed at higher temperature. In this application, the influence of mechanochemical effect on caffeine was evaluated using DSC.
Measurements and Results
The figure below shows the comparison of an original anhydrous caffeine and a ground anhydrous caffeine. Ground anhydrous caffeine was prepared by grinding the sample with a mortar and pestle for 3 minutes. Three milligrams of caffeine materials were weighed in Al sealed pans and heated from room temperature up to 270ºC at a rate of 10ºC/min in an air static atmosphere. The original anhydrous caffeine result reveals an endothermic peak at 160℃ due to phase transition of Form II to Form I. This was followed by another endothermic peak at 237℃ due to the melting of Form I. The ground anhydrous caffeine showed a similar thermal behavior except that the phase transition temperature is observed at 152℃ suggesting that mechanical treatments such as pulverizing have caused a change in thermal behavior of caffeine.
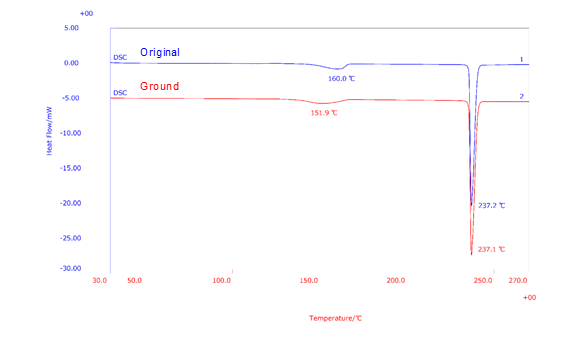
Fig. DSC results of anhydrous caffeine and ground anhydrous caffeine.
Reference
(1): S. Pinto and P. Diogo. J. Chem. Thermodynamics 38 (2006) 1515-1522
(2): A. A. L. Michalchuk, I. A. Tumanov and E. V. Boldyreva. J. Mater Sci 53 (2018) 13380-13389
Instrumentation and software
Thermo plus EVO2 DSC8231
Thermo plus EVO2 Software
RIGAKU RECOMMENDS
DSCvesta

